
mRNA Drug Substance Manufacturing
From blueprint to biomolecule—engineered for stability, perfected from the start, scaled for your program
As your mRNA programs develop further, you will face increasing demands for quantity and quality. To help you advance to the next stage, you need larger lot sizes, higher levels of quality oversight, and consistent lot-to-lot production.
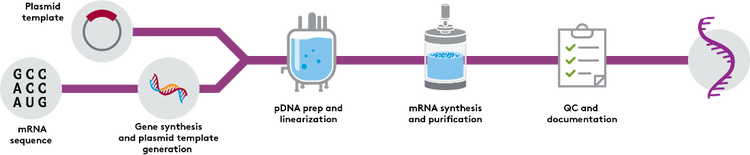
You can rely on Aldevron’s scalable processes to produce high-quality mRNA tailored to your specifications, delivering quantities from milligrams to multi-grams. Additionally, you can access high-quality enzymes and linearized DNA (traditional plasmid DNA or Alchemy™ Cell-free DNA) for your in-house mRNA synthesis needs, ensuring your research and development progress smoothly and efficiently.
Flexible synthesis options
Aldevron supports your mRNA synthesis needs with phase-appropriate options with a variety of polymerases, ranging from wild-type to engineered HiCap and custom-manufactured polymerases. You can benefit from our enzymatic capping services or our partnership with a leading cap analog supplier for us to acquire cap analogs on your behalf. Additionally, we offer both enzymatic tailing and tailing on the template DNA, ensuring your specific requirements are met at every stage of development. Options include:
- Codex® HiCap RNA Polymerase
- Wild-type T7 RNA Polymerase
- Custom manufactured RNA Polymerases (client-specific)
- Wild-type capping/tailing enzymes
- Synthetic cap analogs (supplied by client or another vendor)
- Encoded tailing
Purification
In the final stage of mRNA synthesis at Aldevron, purification is crucial to ensure the quality and efficacy of our products. This process involves multiple steps:
- Chromatography: We use advanced chromatography techniques to separate mRNA from impurities based on their physical and chemical properties. We employ various types of chromatography, such as ion-exchange or affinity chromatography, to achieve the highest purity levels.
- Ultrafiltration (UF)/Diafiltration (DF): Our purification process also includes ultrafiltration and diafiltration methods to further refine the mRNA. Ultrafiltration uses semi-permeable membranes to separate molecules based on size, while diafiltration involves the continuous addition of a solvent to wash away impurities.
These purification steps are essential to prepare the mRNA for the formulation stage, where it will be combined with other components to create the final therapeutic product.
QC testing panel
Ensuring high quality control for RNA production is crucial yet often overlooked early in the development process. Our comprehensive suite of tests addresses critical attributes such as purity, identity, concentration, residual protein, DNA template, endotoxin content, and bioburden. By adhering to USP recommendations and regulatory guidance, we provide unmatched expertise and reliability. Partnering with us means leveraging our established testing capabilities to overcome these significant challenges, ensuring your material meets the highest standards from the start.
Table 1: Quality Attributes and Methods Used for RNA Drug Substance
| Quality Attribute | Attribute | Method |
| Identity | Sequence Confirmation | RT-Sanger Sequencing |
| Identity - UTR | RT-PCR | |
| Content | RNA Concentration | UV spectroscopy (SoloVPE) |
| Potency | Activity Assay | In vitro Transcription Assay |
| Purity/ Integrity | % Intact and fragment mRNA | Capillary Electrophoresis |
| 5’ capping efficiency | IP-RP-HPLC, RP-LC-MS (DNAzyme cut) | |
| 3’ poly(A) (% tailed) | dPCR | |
| 3’ poly(A) length | IP-RP-HPLC (enzymatic tail drop-off) | |
| Impurity | Product Related impurities – dsRNA | ELISA |
| Process Related impurities - Residual Protein and nucleoside | RP-HPLC, RP-LC-MS | |
| Process Related impurities - Residual Template | qPCR |

The Aldevron Advantage
Aldevron offers unparalleled flexibility to develop RNA tailored to your specific program while adhering to regulatory guidelines. We provide a range of options, from the simplest starting materials to the most complex manufacturing operations, ensuring that your unique needs are met at every stage. Our commitment to innovation drives us to support you through each step of the journey. We will find a way to meet the specific needs for your therapy because we believe in it as though it were our own.
RNA design & optimization
Specialized products and services for mRNA design and development that can significantly reduce supply-chain risks, shorten lead times, and enhance quality.
RNA polymerases
Codex® HiCap RNA Polymerase is an innovative, high-performance co-transcriptional capping RNA polymerase engineered to:
- Reduce unwanted immunogenicity by producing less double-stranded RNA
- Cut overall manufacturing costs by more efficiently incorporating cap analogs.
Aldevron-manufactured wild-type T7 Polymerase
Aldevron-manufactured custom RNA polymerases.
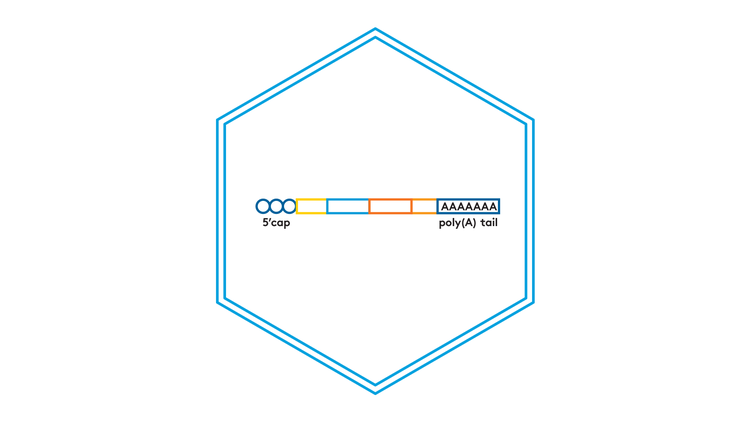
Capping and tailing options
Aldevron-manufactured wild-type capping enzymes
Synthetic cap analogs supplied by clients or other vendors. Allow Aldevron to streamline vendor relations by acquiring TriLink cap analogs on your behalf.
QC testing and regulatory support
Comprehensive testing for critical attributes based on USP recommendations (released in March 2022) and other regulatory guidance.
Having supported over 1,000 clinical trials across modalities, our direct connections and first-hand experience can support you from start to finish, from idea to commercialization, from IND application to clinical trial and beyond.
RNA LNP drug product
Seamless continuation of the RNA drug product manufacturing process incorporating LNP encapsulation, analytical testing, and sterile fill/finish under a single roof and quality system.
Delve into the meticulous process of developing RNA drug substances. Discover how Aldevron ensures purity, quality, and scalability through advanced manufacturing technologies and rigorous quality control measures.
Resources
- mRNA Services (PDF)
- Alchemy™ Cell-free DNA Technology (PDF)
- mRNA Analytical Testing (PDF)
- Streamlining the Pather from Discover to Patient (Webinar)
- Revolutionizing mRNA Manufacturing with Alchemy™ Cell-free DNA Technology (Webinar)
- Mastering the Path: Developing Processes for Successful Clinical-Grade mRNA Manufacturing (Webinar)
- Comprehensive GMP Manufacturing of mRNA-LNP Drug Products: Sequence-to-Vial (Webinar)
- How a Sponsored CMC Platform Accelerates mRNA Projects to the Clinic (Webinar)
- mRNA/saRNA Manufacturing: Considerations to Accelerate the Path to Clinic (Webinar)
- Q&A series with Aldevron CSO, Venkata Indurthi, Ph.D. (PDF)
- Topic 1: Program Development & Support
- Topic 2: Synthesis Process & Methods
- Topic 3: Production & Manufacturing
- Topic 4: Analytics & Testing
- Topic 5: Therapeutic Delivery & Applications