
Nanoplasmid™
A Plasmid Vector System Engineered
for the Edge of Innovation
Nanoplasmid™ Vector System for Gene Delivery Optimization
One vector. Endless possibilities.
Looking to enhance performance and manufacturing from your DNA backbone, without the regulatory concerns? The small Nanoplasmid backbone fulfills all the requirements. The Nanoplasmid Vector System (<500 bp backbone) provides advantages over existing legacy plasmid DNA backbones and minicircles:
- High Transgene Expression
- Increased Edited Cell Yields
- Improve Safety Profile by Eliminating Antibiotic Resistant Gene
- Save Time and Cost-Effective
Combining characteristics of reduced size and the RNA-OUT non-antibiotic resistance selection marker, the platform is ideally suited for a range of therapeutic applications. Aldevron can provide customized Nanoplasmid DNA for each client.
Nanoplasmid Vector Benefits by Applications
| AAV Virus | Lentivirus | Non-viral gene therapy1,2 | DNA vaccines 3 | HDR/CRISPR gene editing4,5 | Transposons6 | MRNA vector protection | |
| Performance | |||||||
| Increase Transgene performance | • | • | N/A | N/A | |||
| Lower Transfection Toxicity | • | • | • | • | • | • | |
| Enhance Manufacturing | |||||||
| Plasmid Template | • | • | • | • | • | ||
| End Product Production | • | • | • | • | |||
| Regulatory Compliant | |||||||
| Yes | • | • | • | • | • | • | N/A |
Have questions? Read our frequently asked questions about Nanoplasmids on our FAQ page.
Nanoplasmid Webinars/Videos
Learn more about Nanoplasmid, view our webinar series here:
- Webinar 1: Advanced plasmid technology: Improving safety and performance
- Webinar 2: Can a smaller plasmid produce huge benefits? The power of small.
- Webinar 3: Genome Editing Tools: Beyond Discovery - Nanoplasmid application in CRISPR/Cas9 based gene editing
- Nanoplasmid: Power of Small Introductory Video
Nanoplasmid Brochure & Literature
Nanoplasmid is a disruptive, episomal technology in the clinical and pre-clinical arena. There have been dozens of publications showing off how this small backbone delivers powerful benefits.
- Boye C, Arpag S, Burcus N, Lundberg C, DeClemente S, Heller R, Francis M, Bulysheva A. Cardioporation enhances myocardial gene expression in rat heart. Bioelectrochemistry. 2021 Dec;142:107892. doi: 10.1016/j.bioelechem.2021.107892. Epub 2021 Jul 27. PMID: 34371349.
Showing improved electroporation transfection methods in vivo and in vitro using Nanoplasmid versus conventional plasmids for naked DNA expression.
- Oh SA, Senger K, Madireddi S, Akhmetzyanova I, Ishizuka IE, Tarighat S, et al. High-efficiency nonviral CRISPR/cas9-mediated gene editing of human T cells using plasmid donor DNA. Journal of Experimental Medicine. 2022;219(5). https://doi.org/10.1084/jem.20211530
This publication showed improved CAR-T knock-in efficiency, reduced cell death, and an improved T cell phenotype for CAR-T cell therapies using Nanoplasmid compared to conventional plasmids and linear DNA as an HDR template.
- Bozza M, De Roia A, Correia MP, Berger A, Tuch A, Schmidt A, et al. A nonviral, nonintegrating DNA nanovector platform for the safe, rapid, and persistent manufacture of recombinant T cells. Science Advances. 2021;7(16). doi: 10.1126/sciadv.abf1333
This article discussed how Nanoplasmid was integrated into a rapid and novel manufacturing workflow for the production of CAR-T cell therapies for patients.
For more information on these and other applications, download the complete bibliography.
LMY-920 for Relapsed/Refractory Myeloma
- Trial ID: NCT05546723
- Phase: 1
- Modality: CAR-T, Transposons (TcBuster)
- Location: US
- Summary: This Phase 1 trial investigates BAFF CAR-T cells for treating relapsed or refractory multiple myeloma.
BAFF CAR-T for Systemic Lupus Erythematosus
- Trial ID: NCT06340750
- Phase: 1
- Modality: CAR-T, Transposons (TcBuster)
- Location: US
- Summary: A Phase 1 trial evaluating BAFF CAR-T cells for autoimmune disease (SLE).
LEGEND Study: EG-70 in NMIBC Patients BCG-Unresponsive and High-Risk NMIBC Incompletely Treated With BCG or BCG-Naïve
- Trial ID: NCT04752722
- Phase: 1 and 2
- Modality: Immune oncology gene therapy
- Locations: US, Australia, Canada, France, Germany, Italy, Korea, Spain, Taiwan
- Summary: EG-70 is a non-viral gene therapy for bladder cancer.
A Clinical Trial of a Prophylactic Plasmid DNA Vaccine for COVID-19 [Covigenix VAX-001] in Adults
- Trial ID: NCT04591184
- Phase: 1 and 2
- Modality: DNA vaccine (direct gene delivery)
- Locations: Burkina Faso, Canada, Senegal, South Africa
- Summary: A DNA-based COVID-19 vaccine using direct gene delivery.
P-BCMA-101 Tscm CAR-T Cells in the Treatment of Patients With Multiple Myeloma (MM)
- Trial ID: NCT03288493
- Phase: 1 and 2
- Modality: CAR-T, Transposons (PiggyBac)
- Location: US
- Summary: This trial used PiggyBac transposon technology for CAR-T therapy.
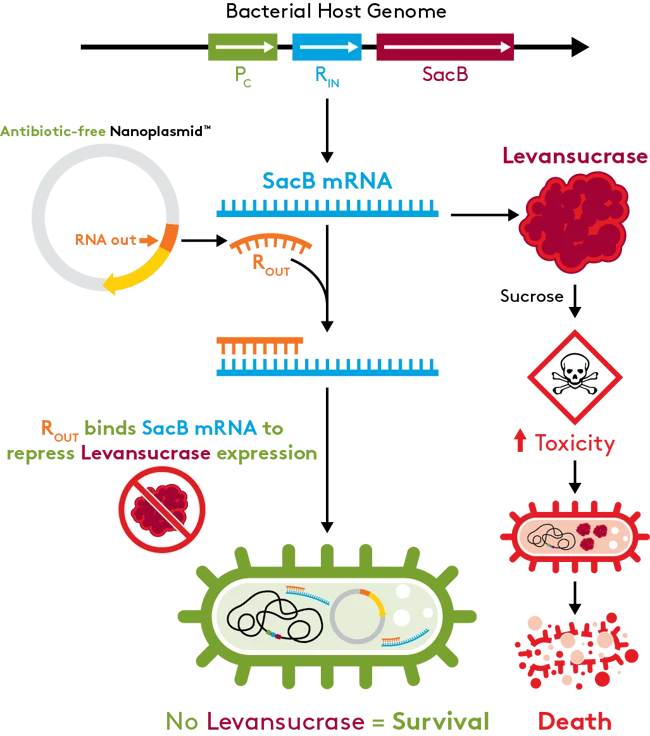
Antibiotic-free selection provided by RNA-OUT
The RNA-OUT platform works by repressing the expression of a counter-selectable marker (SacB) from the host chromosome. SacB encodes for the levansucrase enzyme, which creates a toxic environment in the presence of sucrose, which leads to cell death. Transforming the Antibiotic-free Nanoplasmid in the host expresses a 150bp RNA-OUT antisense RNA (ROUT) that binds the SacB mRNA and represses levansucrase expression, prevents toxicity and to maintain cell survival. The RNA-OUT selectable marker can be used to retrofit existing antibiotic resistance DNA plasmids into antibiotic-free vectors counter-selection system.
Connect with a technical expert
Contact us to schedule a consultation with an expert about your Nanoplasmid product development. In the discussion, our team will evaluate the ability to retrofit your vector into the backbone or design the plasmid within the Nanoplasmid construct for your program.
DISCUSS WITH A NANOPLASMID EXPERT
- Boye C, Arpag S, Francis M, DeClemente S, West A, Heller R, Bulysheva A. Reduction of plasmid vector backbone length enhances reporter gene expression. Bioelectrochemistry. 2022 Apr;144:107981. doi: 10.1016/j.bioelechem.2021.107981.
- Vermeire G, De Smidt E, Geukens N, Williams JA, Declerck P, Hollevoet K. Improved Potency and Safety of DNA-Encoded Antibody Therapeutics Through Plasmid Backbone and Expression Cassette Engineering. Hum Gene Ther. 2021 Oct;32(19–20):1200–1209. doi: https://doi.org/10.1089/hum.2021.105. PMID: 34482757.
- Suschak JJ, Dupuy LC, Shoemaker CJ, Six C, Kwilas SA, Spik KW, et al. Nanoplasmid vectors co-expressing innate immune agonists enhance DNA vaccines for Venezuelan equine encephalitis virus and ebola virus. Molecular Therapy — Methods & Clinical Development.2020;17:810–21.
- Oh SA, Senger K, Madireddi S, Akhmetzyanova I, Ishizuka IE, Tarighat S, et al. High-efficiency nonviral CRISPR/cas9-mediated gene editing of human T cells using plasmid donor DNA. Journal of Experimental Medicine. 2022;219(5). https://doi.org/10.1084/jem.20211530 CC BY 4.0
- Balke-Want H, Keerthi V, Gkitsas N, Mancini AG, Kurgan GL, Fowler C, Xu P, Liu X, et al. Homology-independent targeted insertion (HITI) enables guided CAR knock-in and efficient clinical scale CAR-T cell manufacturing. Mol Cancer. 2023 Jun 26;22(1):100. doi: 10.1186/s12943-023-01799-7.
- Pomeroy EJ, Lahr WS, Chang JW, Krueger J, Wick BJ, Slipek NJ, et al. Non-viral engineering of CAR-NK and CAR-T cells using the TC buster transposon system™. bioRxiv 2021.08.02.454772; doi: https://doi.org/10.1101/2021.08.02.454772 23-Nanoplasmid-
