
mRNA-LNP Drug Product Manufacturing & Fill/Finish
Aldevron delivers experience
Aldevron is a global partner offering a flexible end-to-end sequence-to-vial service workflow that accelerates the development and manufacturing of RNA therapeutics. Aldevron enables faster delivery by accelerating the timeline by approximately 50%, which reduces the risk in high-quality RNA drug substance and drug product manufacturing with lipid nanoparticle (LNP), analytical testing, and sterile fill/finish under one facility and a single quality system. We have delivered high-quality mRNA for our clients since 2017 with >100 cGMP batches released, and supporting numerous IND submissions through our dedication to:
- Being a seasoned pioneer
- Committing to quality
- Easing complexity to our clients
Advancing with Aldevron
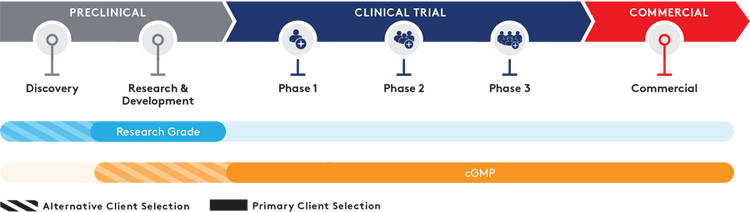
Quality grades
Your goal is to change lives with a successful therapy or vaccine. There are many steps between your current program and that end goal. Working with a partner who can support you through the whole journey can significantly improve your chance of success. Aldevron offers research-grade and full cGMP manufacturing services at scales and a complete range of process development services required to support your project through each stage from discovery to clinical trials and commercialization.
Technical Expertise
Aldevron provides a range of support during the process of high-quality mRNA drug product to your specifications using scalable and processes capable of delivering milligram to multi-gram quantities from sequence to vial. For your project requirements, we can customize the specific solutions with a range of tools to streamline your mRNA program.
- RNA backbones and NanoplasmidTM backbone
- DNA template generation/optimization
- Plasmid DNA Production, purification, and linearization
- mRNA synthesis with in vitro transcription, capping and purification
- Analytics - Method development and validation
- Quality control and regulatory standards
- Lipid nanoparticles (LNP) encapsulation and fill/finish with agnostic technology
We maintain consistent manufacturing conditions through all stages of the drug product process necessary to scale the mRNA drug substance and the signature nanoparticle formation to provide reproducible GMP-compliant mRNA-LNP manufacturing.
You can learn more by watching our webinar detailing our RNA-LNP drug product service under a single ecosystem. We demonstrate our scalable manufacturing capabilities within our dedicated facilities under a single quality workflow.
Drug product production
Your success is important to us, and our mRNA process optimization and manufacturing is complemented by decades of experience in plasmid DNA and enzyme development. Over time, we’ve found that one size does not fit all. Working with our Cytiva and Acuitas partners to be technology agnostic, we are prepared to support any or all of your mRNA/saRNA development from template design through encapsulation and fill/finish. We have many years of experience being technology agnostic to meet our clients’ specific needs. Therefore, we accept technology transfer at any stage of the mRNA drug product process and customize each project to fit each product's specific challenge, goals, and objectives.
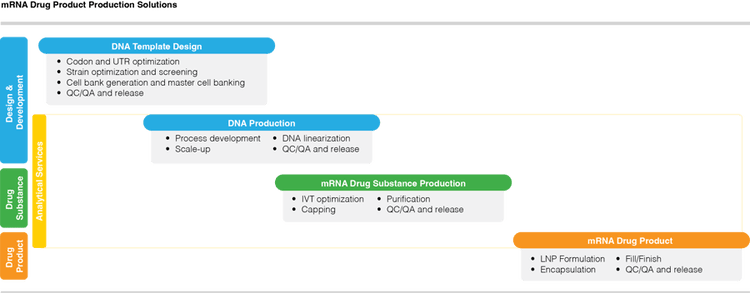
Learn more about each step in the complete solution for mRNA drug development and manufacturing process:
DNA Template Design & Production |
mRNA Drug Substance Production |
mRNA Drug Product
|
| Optimization, screening, and development of a master cell bank for production of high-performance linearized plasmid template DNA for in vitro Transcription. | A flexible system using Aldevron-made IVT enzymes, enzymatic or co-capping, and a purification strategy based on user requirements. | LNP encapsulation is done with the support of LNP encapsulation expertise. Closed aseptic filling of mRNA drug products that reduces risk and improve agility. |
Learn More |
Learn More |
Learn More |
- BROCHURE: mRNA Manufacturing
- WEBINAR: Comprehensive GMP Manufacturing of mRNA-LNP Drug Products: Sequence-to-Vial
- WEBINAR: How a Sponsored CMC Platform Accelerates mRNA Projects to the Clinic
- WEBINAR: mRNA/saRNA Manufacturing Webinar: Considerations to Accelerate the Path to Clinic
- INTERVIEW: Q&A series with Aldevron CSO, Venkata Indurthi, Ph.D.
- Topic 1: Program Development & Support
- Topic 2: Synthesis Process & Methods
- Topic 3: Production & Manufacturing
- Topic 4: Analytics & Testing
- Topic 5: Therapeutic Delivery & Applications
- Cloning Backbones – Royalty and license free empty vectors contain the T7 promoter region for RNA production, with an option for encoded poly(A) available in one construct.
- In vitro transcription & capping enzymes – Available as standard, in-stock products or custom manufactured to meet specific client requirements.
- Nanoplasmid™ vectors – A next-generation, small cloning backbone that produces high-yield linear plasmid DNA templates by stabilizing the polyA tail.
- DasherGFP mRNA – Study transfection and expression with an mRNA encoding an IP-free fluorescent protein which mimics mature mRNA with 5’ Cap 1 structure and 3’ poly(A) tail.
Facilities

Breakthrough Campus
Fargo, ND
Plasmid DNA, mRNA, Protein manufacturing at cGMP & GMP-Source™ quality
Get in Touch with an Expert
| region | na1 |
| portalId | 1769030 |
| formId | 0cf398d8-651d-4ebd-a68e-224851d5ab8d |
| target | services-hubspot-form |


