
pALD-AAV System
In Stock, Royalty Free, Plasmids and Cloning Vectors for rAAV Manufacturing
For the past 25 years, researchers and clinicians on the cutting edge of cell and gene therapy applications have been forced to create custom solutions for almost every challenge. But, as the industry matures, years of developing products and processes have revealed a pathway to standardization. Balancing variables such as availability, quality, performance, and reproducibility, Aldevron offers the pALD-AAV system, with standard rep/cap plasmids, 2 options for the helper plasmid, and transgene cloning vectors.
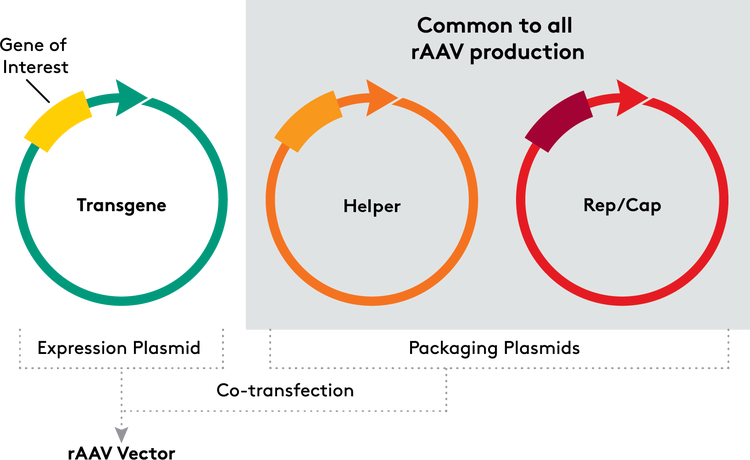
pALD-AAV system advantages:
- Packaging plasmids in stock at research grade, GMP-Source™, and cGMP (varies by plasmid)
- No royalties charged by Aldevron for research through commercial use of the pALD-AAV system
- Kanamycin resistance
- Consistent, reliable supply
pALD Helper Plasmids – pALD-X80 and pALD-HELP
Aldevron offers two inventoried helper plasmids, pALD-X80 and pALD-HELP, for use in recombinant adeno-associated virus (rAAV) manufacturing. Both can replace helper plasmids in existing AAV manufacturing protocols. pALD-X80 has a longer history, including more clinical trials, and has a Drug Master File (DMF) on file with the US FDA. Alternatively, pALD-Help removes extraneous DNA, including a Hexon/fiber coding sequence, improving its safety profile and manufacturing efficiency to lower costs.
| PALD-X80 | PALD-HELP | |
| Size | 18.9 kb | 11.6 kb |
| Kanamycin antibiotic resistance | ✔ | ✔ |
| Licence and royalty free for research through commercial applications | ✔ | ✔ |
| Partner group | Ask Bio | None (Aldevron owned) |
| Hexon/fiber sequence free | No | ✔ |
| History of use in clinical trails | ✔ | No |
| Immediately available at Research Grade | ✔ | ✔ |
| Immediately available at GMP-Source® | ✔ | ✔ |
| Immediately available at cGMP | ✔ | ✔ |
Performance
pALD-X80 Expression Data for rAAV Luciferase
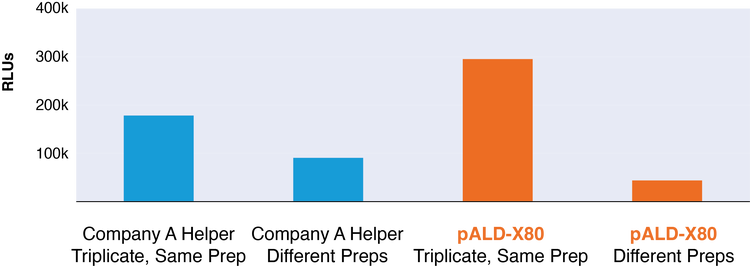
Figure: Transduction data from AAV2 viral vector with luciferase transgene indicating performance of pALD-X80 relative to commercially available helper plasmid.
pALD-HELP, pALD-AAV Rep/Cap Infectious Titer Data
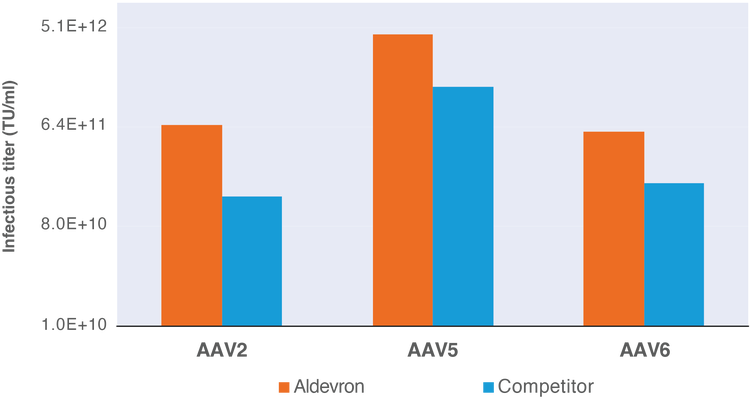
Figure: Infectious titer data for rAAV vector production in adherent HEK293T cells using pALD-HELP and pALD-AAV rep/cap plasmids as compared to commercially available rep/cap plasmids.
Order below or request a free sample of the research grade plasmids, try them, and see which is best for your application.
pALD-AAV Rep/Cap Plasmids
There are many variations of the rep/cap plasmids being used in AAV therapies. We offer a small set of rep/cap plasmids containing the gene sequence for AAV serotype 2 replication (rep) protein paired with either serotype 2, 5 or 6 capsid (cap) protein respectively. Use of AAV serotype 2 rep proteins in combination with other serotypes for cap proteins is referred to as pseudotyping, which provides enhanced and refined tissue tropism in some applications.
| Plasmid | Rep Serotype | Cap Serotype | Pack Size |
| pALD-AAV2 | 2 | 2 | 10 mg |
| pALD-AAV5 | 2 | 5 | 10 mg |
| pALD-AAV6 | 2 | 6 | 10 mg |
Clients may submit other rep/cap serotypes/pseudotypes for custom manufacturing .
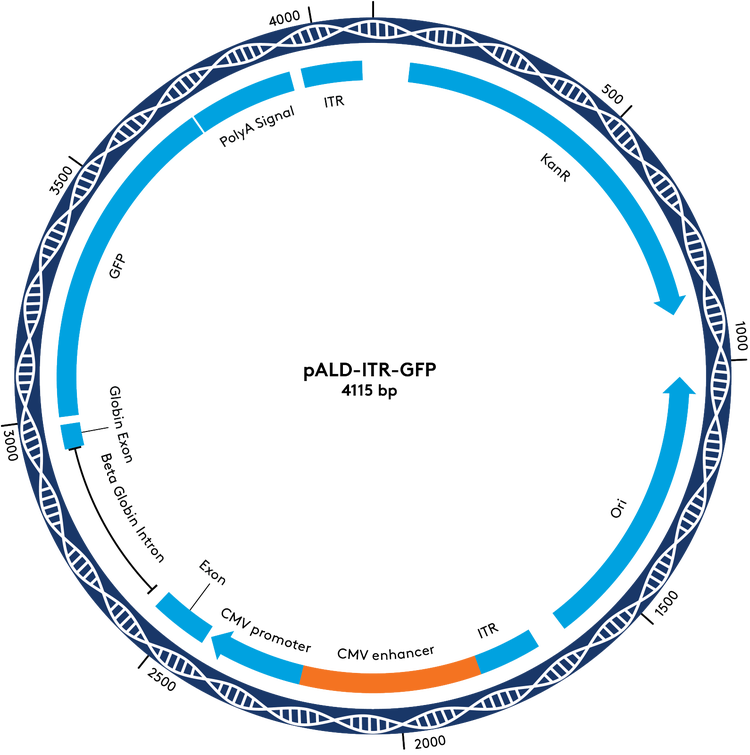
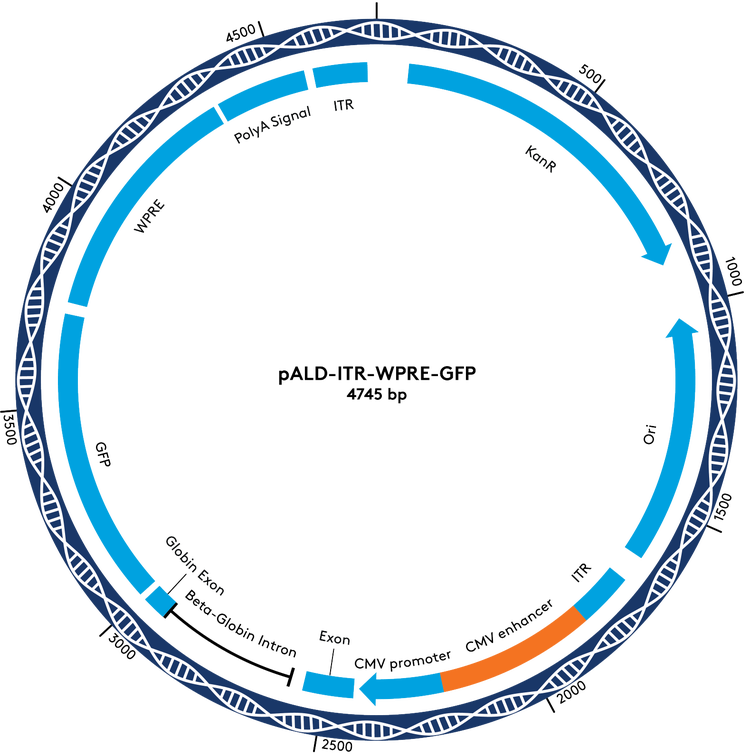
pALD-ITR Cloning Vectors & Reporters
For the portion of the pALD-AAV system that cannot be standardized, we offer a set of cloning vectors, with and without WPRE, as an opportunity to move your project forward more efficiently. These cloning vectors have stabilizing ITRs that work well in Aldevron’s platform manufacturing system. To further simplify your program development, we have GFP reporter versions of these vectors available for testing and optimization.
Transgene plasmids are custom manufactured to meet your requirements.
Interested in other cloning backbones? Check out pALD Cloning Backbones.
pALD-AAV System Resources
| pALD-AAV System Quality Control Specifications (excludes pALD-Cloning Vectors) |
pALD-ITR Cloning Vectors Quality Control Specifications | |
| Appearance | Clear and Colorless | Clear and Colorless |
| Buffer | TE | TE |
| USP Sterility <71> * | No Growth | N/A |
| Bioburden | No growth in 48 hours at 37 +/- 2 C | N/A |
| Concentration | 1.0mg/mL +/- 10% | 1.0mg/mL +/- 10% |
| DNA Homogeneity | ≥80% Supercoiled | ≥80% Supercoiled |
| Endotoxin | ≤100 EU/mg | ≤100 EU/mg |
| Identity | Confirmed Against DNA Ladder | Confirmed Against DNA Ladder |
| Plasmid Identity | Identical to Reference Sequence | Identical to Reference Sequence |
| ABS 260/280 Ratio Purity | 1.80 – 2.00 | 1.80 – 2.00 |
| Residual Host Genomic DNA via EtBr Gel | N/A | ≤5% |
| Residual Host Genomic DNA via qPCR | ≤5% | N/A |
| Residual Host Protein via BCA | ≤2% | N/A |
| Residual Host RNA via SYBR Gold | ≤5% by Semi-Quantitative Densitometry | N/A |
| Residual Host RNA via EtBr Gel | N/A | Not Visible at 200 ng Load |
| Restriction Digest | Matches Expected Restriction Pattern | Matches Expected Restriction Pattern |
| Mycoplasma Contamination | Negative for the Presence of Mycoplasma | N/A |
*Sterility testing is not available for research grade material. Bioburden by direct inoculation with a specification of no growth after 48 hours is performed at the research grade level (excludes pALD-ITR Cloning Vectors).
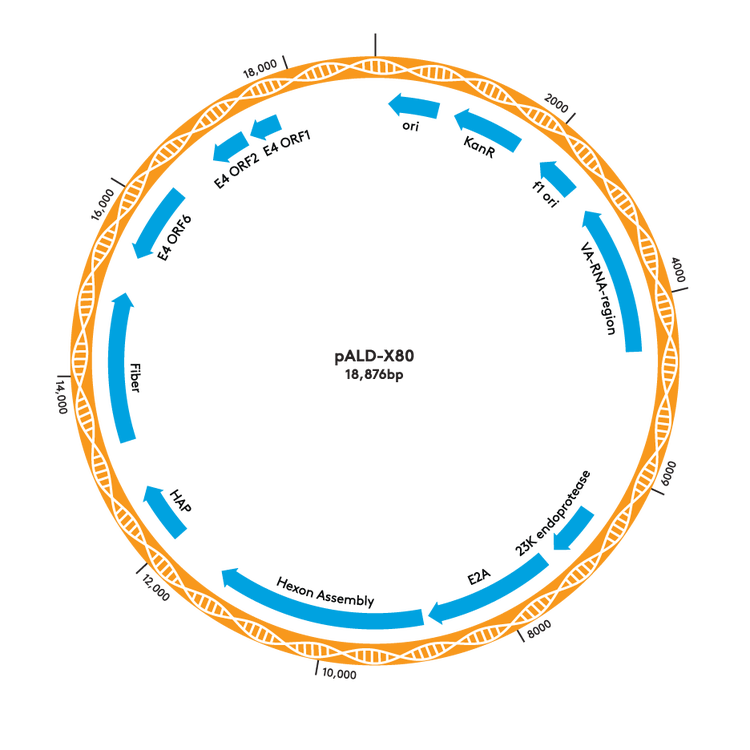
pALD-AAV System Plasmid Maps
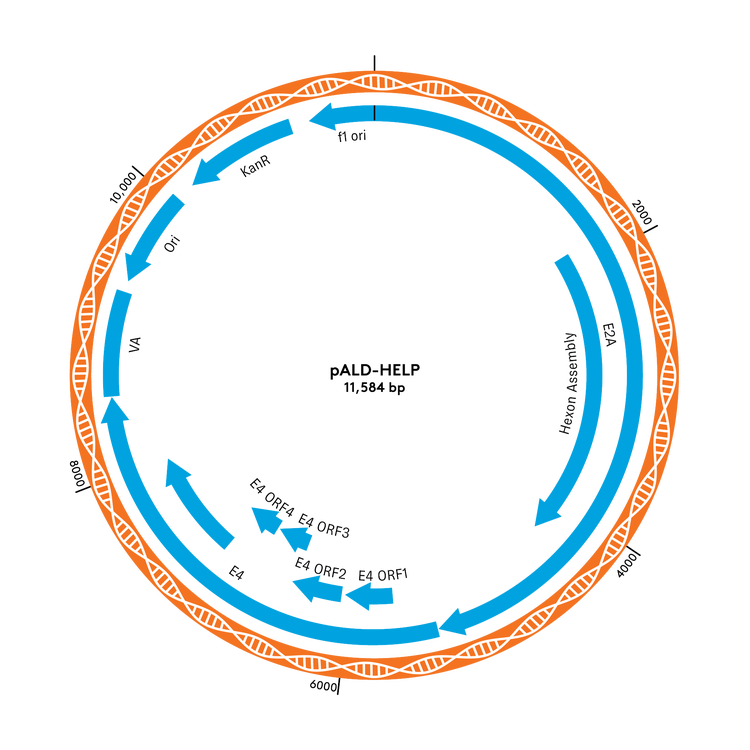
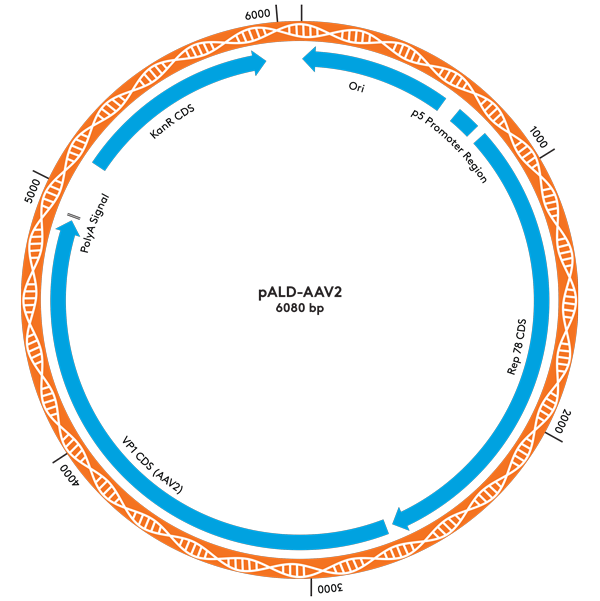
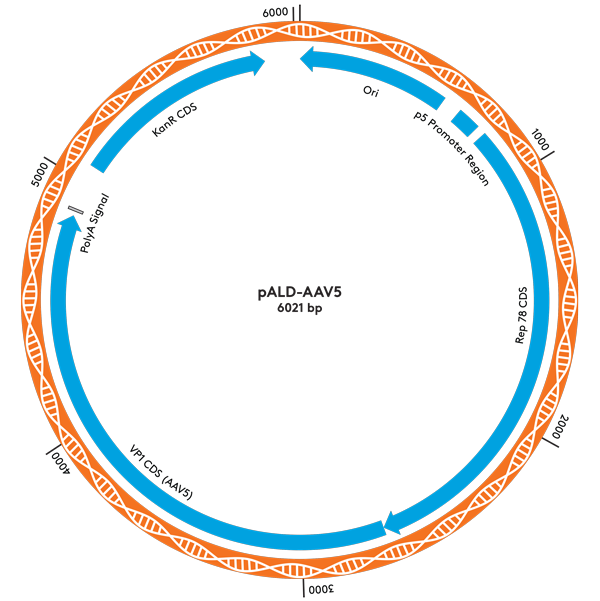
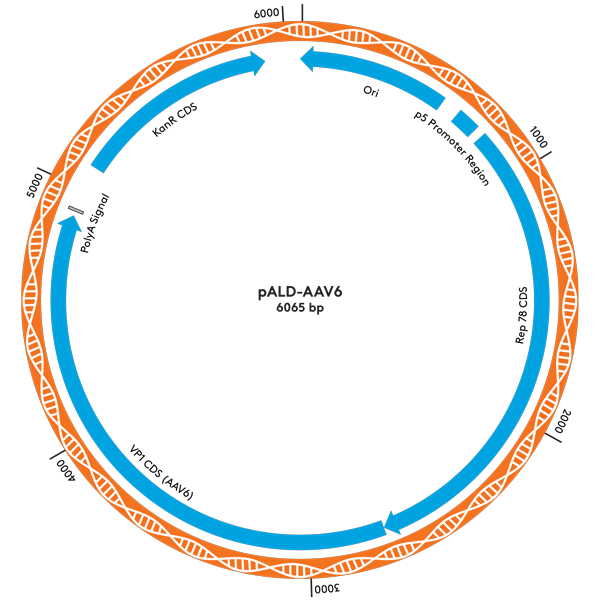
Ordering Information
| Product | Pack Size | Catalog Number | RG Purchase Option |
| pALD-X80 - RG | 10 mg | 5017-10 | Buy Now |
| pALD-HELP - RG | 10 mg | 5082-10 | Buy Now |
| pALD-AAV2 - RG | 10 mg | 5057-10 | Buy Now |
| pALD-AAV5 - RG | 10 mg | 5058-10 | Buy Now |
| pALD-AAV6 - RG | 10 mg | 5059-10 | Buy Now |
| pALD-ITR-WPRE-GFP - RG | 10 mg | 5069-10 | Buy Now |
| pALD-ITR-GFP - RG | 10 mg | 5062-10 | Buy Now |
