
GMP-SourceTM Grade Plasmid DNA
Cost-effective, faster alternative to full cGMP plasmid DNA
As your project matures from research to early phase clinical trials you will need higher quality plasmid or NanoplasmidTM DNA. You will have to balance budget, timelines, and your risk tolerance to find the best quality grade for your needs. To help balance those needs, Aldevron pioneered GMP-SourceTM, a faster, cost-effective alternative to full cGMP manufacturing.
GMP-Source can be manufactured to standard or customizable specifications. Adopting the most relevant features of cGMP manufacturing, including traceability, document control and materials segregation, GMP-Source can be used as an ancillary or critical raw material for constructs used in clinical trials.
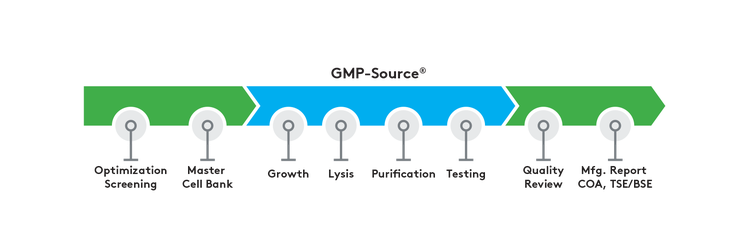
GMP-Source manufactured plasmid and Nanoplasmid DNA products offer:
- Screening for optimal growth conditions
- Growth via shake flask or high-density fermentation
- Alkaline lysis
- Chromatographic purification
- Consistent manufacturing process
- Certificate of Analysis (CoA)
- E. coli master cell bank generation
- Manufacturing summary report
- TSE/BSE statement
- Master Batch Records dictate process (controlled by Aldevron)
- ISO classified fill/finish with environmental monitoring
- QA oversight
Some clients choose GMP-Source to support pre-clinical and early phase trials. The data generated from a GMP-Source campaign can be utilized to assist the transition to full cGMP for later clinical trial phases. The GMP-Source process is so closely mapped to full cGMP that the transition is a smooth one.


