
Trusted Partner for Flexible & Scalable Custom Protein Manufacturing
Customized Protein Manufacturing: Your Path to Success
When it comes to protein manufacturing, projects are as unique as our client’s aspirations. The challenges, from construct design and process development through analytical testing and characterization are equally unique. To bring your custom diagnostic and therapeutic proteins to life, it's important that your CDMO partner understands the power of customization, offering you a combination of capabilities to fulfill your specific requirements.
- Flexible process development or tech-transfer of existing methods
- Dedicated project management team
- Custom assay development that supports product release testing
- Product-specific quality control analysis
- Manufacturing Batch records tailored for each custom protein
- Progressive Quality Levels: Research Grade, Diagnostics, Therapeutic
Working With Aldevron
We have decades of experience handling small to large projects, ensuring quality, purity, and reliability in dedicated facilities with all the necessary controlled environments and the meticulous validation processes in place. We have immediate capacity at our newly expanded facility to get your project in now*.
- Expertise and capability. Decades of experience with protein expression, purification and analytical characterization.
- Flexibility and timing. Agility to scale up or down within the timeline of your project. Our facility can get your project in immediately and deliver on your timeline*.
- Track record with regulations and documentation. Extensive interaction with regulatory agencies.
- Project management and communication. Dedicated project managers effectively manage critical processes and communication.
- Quality. We deliver consistent high-quality proteins, batch to batch, to help ensure your project succeeds from start-to-finish.
- State-of-the-art facilities. Our purpose-built facilities support our clients from the start of their research, in the clinic, or commercial production.
Therapeutic Protein

- Manufactured at the Breakthrough Campus** in Fargo, North Dakota
- Track record with GMP regulations (21CFR210 and 211) and documentation
Diagnostic Protein

- Manufactured at the Innovate Campus in Madison, Wisconsin
- QMS certified to ISO 13485:2016 (21CFR820)
**This manufacturing facility was visited by the Center for Biologics Evaluation & Research (CBER) in November 2021. CBER conducted a Pre-License Inspection (PLI) and a general GMP audit of Aldevron, LLC. No Form FDA 483 was issued, and the inspection was classified as no action indicated (NAI)
*Availability as of July 2023 for process development, tech transfer, and manufacturing for research and diagnostics at the Innovate campus. Subject to change without notice.
Development Process
We have an experienced team of dedicated client support specialists, project managers and senior scientists to create a plan and guide product development work, ensuring that the methods are suitable for the client’s application. Process optimization ensures a seamless transition to our manufacturing group. Once development is complete, clients may elect to scale manufacture of products in either our ISO 13485 certified facility or cGMP facility.
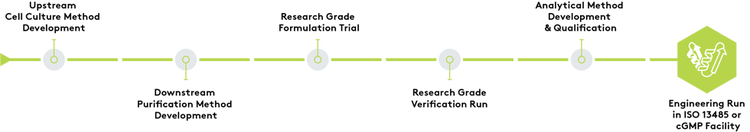
Transfer of Existing Process
Clients interested in protein manufacturing in support of clinical, diagnostic, and commercial applications have the option of transferring an existing process into Aldevron. Clients choosing to transfer an existing method will engage with Aldevron’s protein manufacturing and process optimization subject matter experts. Process optimization may be recommended to ensure a seamless transition. Regardless of the path taken, Aldevron’s internal manufacturing readiness process ensures the successful transition and execution of both the engineering and manufacturing runs.

The Aldevron Advantage - Agility & Right-sized to Respond Quickly
A good partner can help keep a project moving forward by sharing best practices and working with their clients on flexible manufacturing strategies to meet your therapeutic and diagnostic product needs. We can put together a detailed statement of work that adheres to a client’s specifications while sticking to a realistic deadline. Whether you have interests in protein upstream and downstream processing or analytical services, our experienced team of professionals can work with you through every step from pre-clinical to GMP.
- Aldevron has expertise with:
- Construct design
- Codon optimization and gene synthesis
- Fully characterized GMP master cell banks
- Scalable process design and optimization.
- Utilization of single-use fermentation technologies.
- Aldevron has extensive experience with:
- Developing, optimizing, and scaling-up traditional modes of chromatography including both chromatographic media and membrane formats.
- Adoption and implementation of modern scalable purification technologies to increase client process productivity.
In addition to the assays below, we can provide custom developed procedures to meet your requirements.
| Assay | Method |
| Appearance | Visual |
| Purity, Protein-related | Gel electrophoresis (SDS-PAGE) |
| Purity, Protein-related | Reversed-Phase chromatography (RP-HPLC) |
| Aggregation | Size-exclusion chromatography (SEC-HPLC) |
| Concentration | UV spectroscopy |
| Residual Host Cell DNA | PCR, quantitative |
| Residual Host Cell Proteins | ELISA |
| Endotoxins | USP <85> |
| Microbial Enumeration | USP <61> |
Talk with our experts
Interested in working together or have questions about our products and services? Reach out to our client relations team to learn more about what we offer and how to get started on your next project.
| region | na1 |
| portalId | 1769030 |
| formId | 715824d6-fe12-4748-86be-0d19377eeb97 |
| target | Services-hubspot-form |