
pALD Lenti Products
Lentiviral Vector Plasmids
A solution from early research through commercial development
Aldevron provides pALD-Lenti packaging plasmids through our partnership with OXGENE, who have optimized them for lentiviral vector production. They are immediately available at research grade, GMP-Source™, and cGMP. This will potentially reduce your cost and time to manufacture vectors for clinical trials.
The Aldevron pALD-Lenti system has several advantages:
- Immediately available at Research Grade, GMP-Source and cGMP
- No royalties charged by Aldevron for research through commercial use of plasmids for lentiviral manufacture
- Demonstrated performance producing lentiviral vectors
Provided under license from:

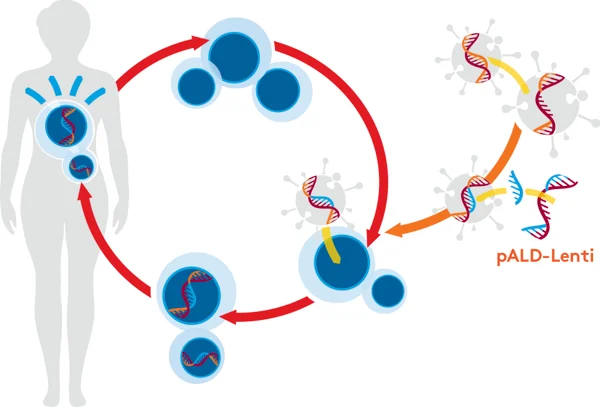
pALD-Lenti System
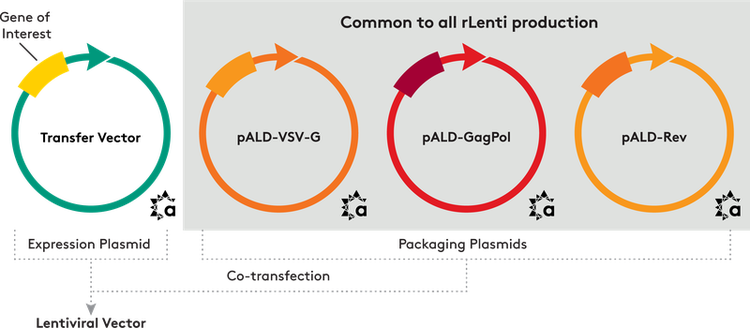
What's included
We provide three packaging plasmids and an expression plasmid, including:
- pALD-Lenti-EGFP
- pALD-VSV-G
- pALD-GagPol
- pALD-Rev
We can clone your gene of interest into the pALD-Lenti-EGFP expression plasmid, replacing the EGFP sequence.
The system has been optimized and represents the state of the art in lentiviral vector production, including:
- Codon optimization
- Vector backbones minimized
- Minimization of homology with HIV/VSV
- Minimization of inter-cassette homology
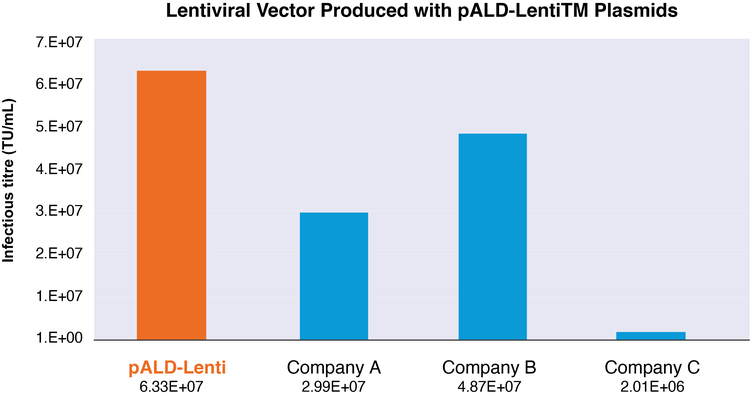
In addition to the optimized design, lentiviral vectors produced with the pALD-Lenti system transfect cells with a higher infectious titer than commercially available kits.
- Download the label license for use of pALD Lenti products
- Download a non-confidential transfection protocol without plasmid ratio information
- Request access to sequences and confidential transfection protocol
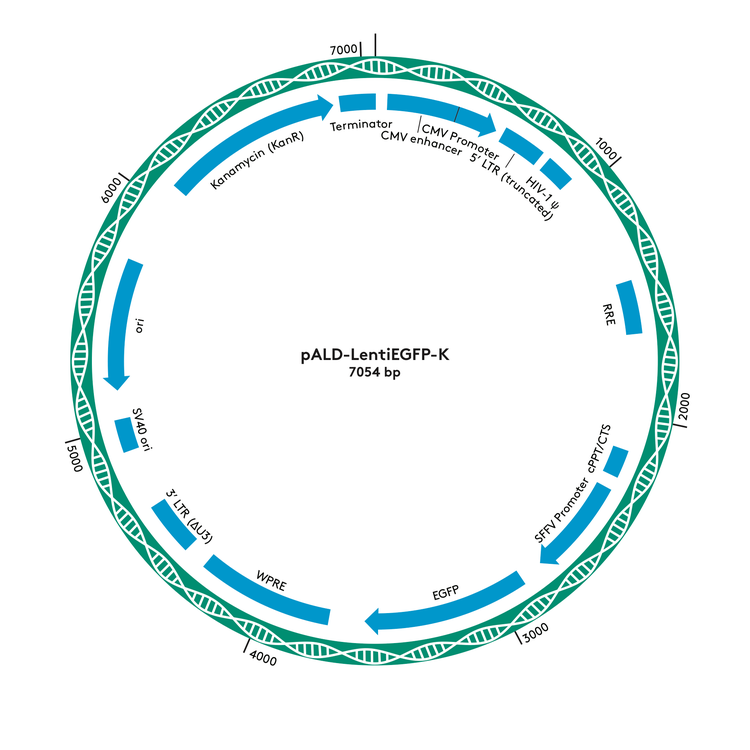
pALD-Lenti Plasmid Maps
pALD-LentiEGFP-K
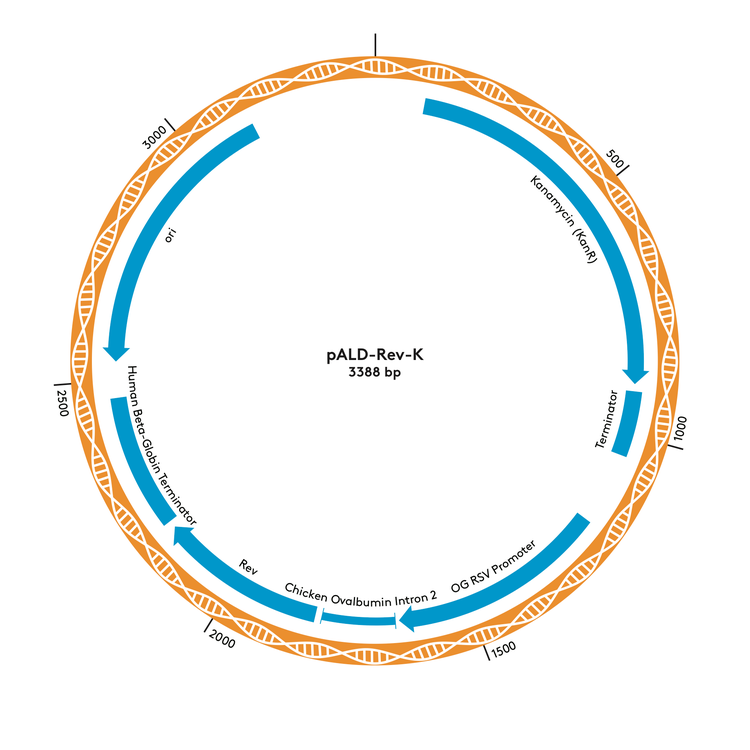
pALD-Rev-K
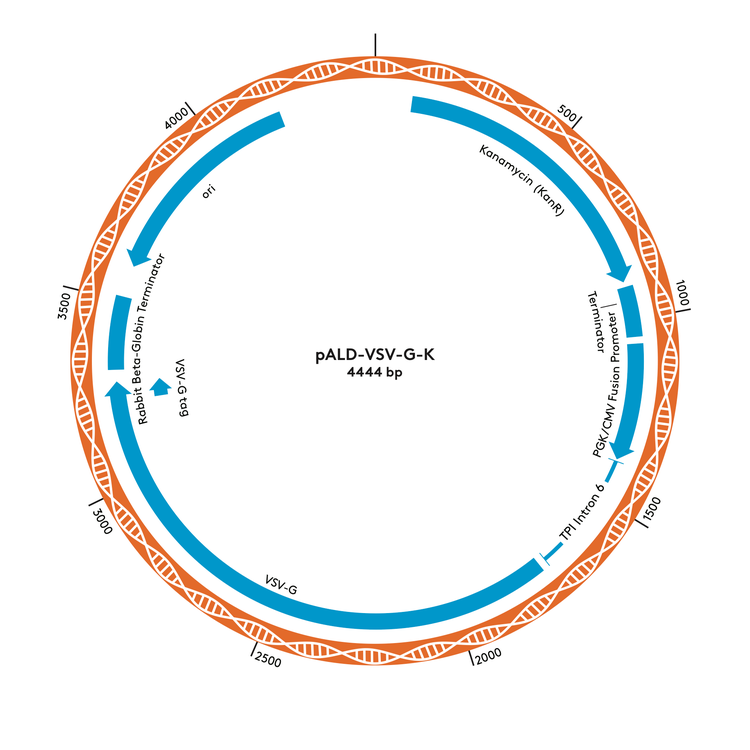
pALD-VSV-G-K
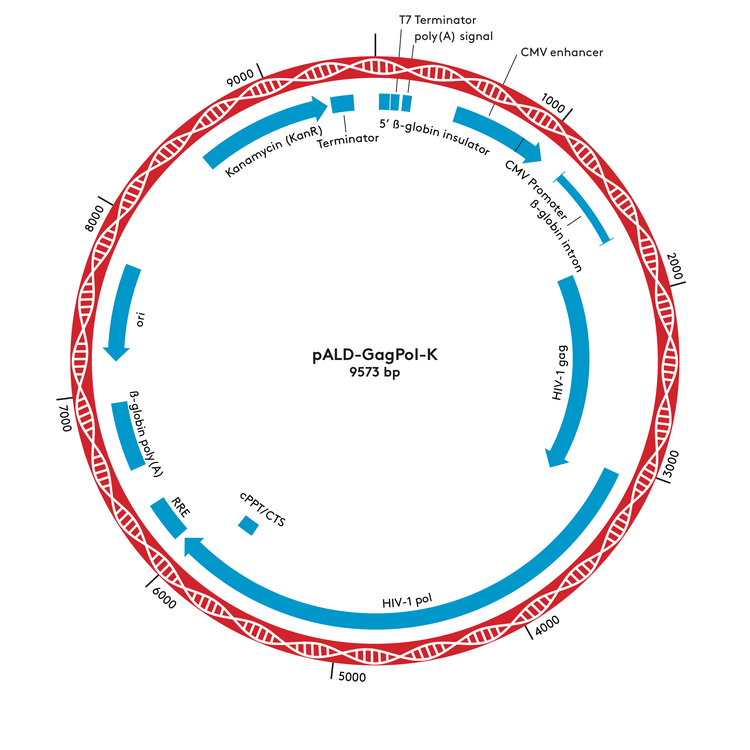
pALD-GagPol-K
| Appearance | Clear and Colorless |
| Buffer | TE |
| USP Sterility <71> * | No Growth |
| Concentration | 1.0 mg/mL +/- 10% |
| DNA Homogeneity | ≥ 80% Supercoiled |
| Endotoxin | ≤ 100 EU/mg |
| Identity | Confirmed Against DNA Ladder |
| Plasmid Identity | Identical to Reference Sequence |
| ABS 260/280 Ratio Purity | 1.80 - 2.00 |
| Residual Host Genomic DNA via qPCR | ≤ 5% |
| Residual Host Protein via BCA | ≤ 2% |
| Residual Host RNA via SYBR Gold | ≤ 5% by Semi-Quantitative Densitometry |
| Restriction Digest | Matches Expected Restriction Pattern |
| Mycoplasma Contamination | Negative for the Presence of Mycoplasma |
*Sterility testing is not available for research grade material. Bioburden by direct inoculation with a specification of no growth after 48 hours is performed at the research grade level.
Research Grade (RG) Ordering Information
| Product | Pack Size | Catalog Number | RG Purchase Option |
| pALD-LentiEGFP-K - RG | 5 mg | 5030-5 | Buy Now |
| pALD-LentiEGFP-K - RG | 10 mg | 5030-10 | Buy Now |
| pALD-Rev-K - RG | 5 mg | 5032-5 | Buy Now |
| pALD-Rev-K - RG | 10 mg | 5032-10 | Buy Now |
| pALD-VSV-G-K - RG 5 mg | 5 mg | 5036-5 | Buy Now |
| pALD-VSV-G-K - RG 10 mg | 10 mg | 5036-10 | Buy Now |
| pALD-GagPol-K - RG | 5 mg | 5034-5 | Buy Now |
| pALD-GagPol-K - RG | 10 mg | 5034-10 | Buy Now |

