
Aldevron Breakthrough Blog
Supporting AAV and Lentiviral Vector Development and Commercialization
June 9, 2020 / by Aldevron
Plasmids are essential for the development of viral vectors used to manufacture novel gene therapies and viral vaccines. Aldevron is supporting the innovation of drug developers in this space by providing standardized royalty-free, bulk AAV helper and lentiviral packaging plasmids for research and GMP production.

IMPORTANCE OF PLASMIDS
Plasmids are circular DNA molecules found mainly in bacteria, but also in yeast and plants, that replicate independently from the host’s chromosomal DNA and enable bacteria to transfer genetic information from one to another via the process of horizontal gene transfer. They may also serve other functions, such as imparting antibiotic resistance. Natural plasmids contain an origin of replication, which controls the host range and copy number of the plasmid and allows initiation of replication. They also generally have at least one other gene that facilitates bacterial survival. Engineered plasmids are designed to introduce foreign DNA into other cells — typically mammalian cells during biopharmaceutical manufacturing. This process is referred to as plasmid transfection. Like natural plasmids, engineered plasmids (also referred to as vectors or constructs) with specifically inserted genes have an origin of replication and a selection marker (e.g., antibiotic resistance to allow for the selection of plasmid containing bacteria) and cloning site(s). Expression plasmids also have promoter regions that determine which cell types the gene is expressed in and the amount of recombinant protein produced. Once the desired vector has been created, it is transformed into bacterial cells that are then selectively grown on antibiotic plates. The type of plasmid — cloning, expression, gene knockdown, reporter, viral and genome engineering — dictates the end-use application, including the production of viral vectors for gene therapies and viral vaccines.
TRIPLE-TRANSFECTION PROCESS
There are several methods for manufacturing viral vectors. One popular technique involves the use of an insect cell/baculovirus expression system. Another system, which relies on a set of engineered herpes simplex viruses (HSVs) and a mammalian cell line, also has advantages, but is patent-protected and must be licensed. A producer cell method involves integration of all necessary genes into the genome of the cell, which allows for very stable expression but is highly complex and is generally only selected when one needs to manufacture a large quantity of a specific viral vector. The most common plasmid transfection approach used for adenovirus (AV) and adeno-associated virus (AAV) vector production is transfection in mammalian cell lines, typically HEK293 cells. Multiple different plasmids are required in this method. For AAV, the cis-plasmid contains the gene of interest flanked by inverted terminal repeats (ITRs), which allow the genome to infect cells and then express the gene of interest. The trans-plasmid (also known as the Rep-Cap plasmid) contains the Rep and Cap AAV genes, which are not sandwiched between the two ITRs. The third plasmid — called the helper plasmid — contains the E4, E2a and VA genes for AAV (or AV), with the cell itself providing the AV E1A gene. As a result, no co-infection with adenovirus is required.
The cis-plasmid is unique for each recombinant AAV vector to be produced, as it contains the specific transgene of interest. The trans-plasmid containing the Rep and Cap genes is specific to the serotype of AAV being used. Different serotypes elicit a distinct immune response and also can have different tissue tropisms. The helper plasmid, however, is always the same regardless of the transgene and serotype. In some cases, the trans-plasmid and helper plasmid have been combined into one larger plasmid, allowing for a two plasmid system. This approach has some advantages, most notably a higher transfection rate, since only two plasmids need to enter the cell rather than three. There are potential cost savings to this method as well, but the combined plasmid is very large and more complex to produce. This approach tends to be limited to projects that involve only certain serotypes.
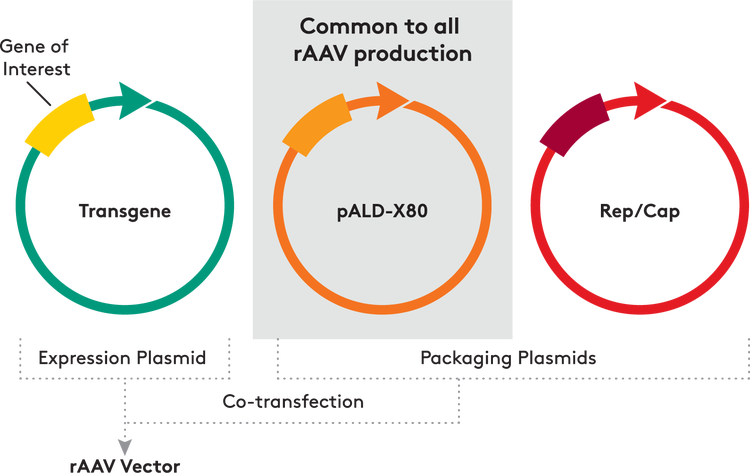
BULK AAV HELPER PLASMID
As a leading contract manufacturer of plasmids, Aldevron has extensive experience producing the three types of plasmids at research to commercial scale. Because the helper plasmid is the same for all triple-transfection projects, it is produced on a regular basis. Part of Aldevron’s growth strategy is to advance the field by becoming a full-service company and adding value for clients through various product offerings. Production of bulk helper plasmid as a standard, off-the-shelf product for research, clinical trial material production and commercial manufacturing is an ideal offering for Aldevron. Our first focus was on the AAV therapy space. Two types of helper plasmids are used in AAV vector production: AAV AdΔF6 and AAV pXX6-80. There are various forms of each available that have various optimizations to perform better during transformation and viral vector production. Aldevron collaborated with Asklepios Biopharmaceutical, Inc. (AskBio), a gene therapy company, to make its helper plasmid available as an off-the-shelf product. AskBio’s pXX6-80 plasmid has been used in recombinant AAV (rAAV) production since 1991 and has been safely used in viral vectors administered to humans. In addition, Aldevron has produced all of AskBio’s clinical GMP material and is familiar with the production of this helper plasmid. Furthermore, both companies are dedicated to advancing gene therapy with enabling technologies that get treatments to patients as quickly as possible. Our pALD-X80 standardized helper plasmid relies on kanamycin resistance rather than ampicillin resistance, which is commonly found in older helper plasmids. Many people have allergic reactions to ampicillin, and histamine responses can occur even with ampicillin reduced to very low levels. As a result, regulatory agencies are demanding that viral vector manufacturers avoid ampicillin resistance. Another crucial element of this product offering is the agreement that no royalty fees would be passed on to Aldevron’s customers; they only pay for the pALDX80 product itself. Given the intellectual property minefield in the gene therapy space, this approach helps dramatically simplify the use of the off-the-shelf material. Aldevron also makes small quantities of pALD-X80 available for free to academic and pharmaceutical industry researchers investigating its use for viral vector production.
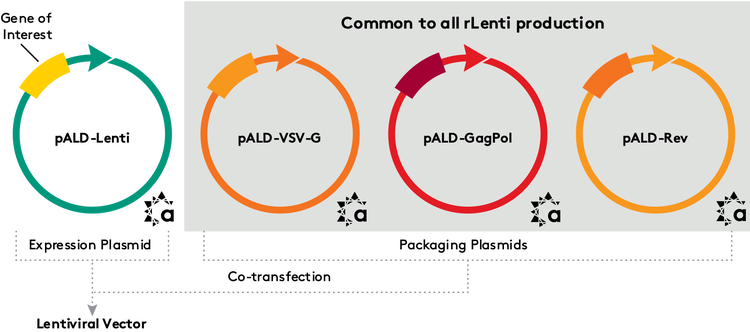
STANDARDIZED LENTIVIRAL PACKAGING PLASMIDS
In the production of lentiviral vectors, the number and types of plasmids used differ, but the overall manufacturing process is similar. The process is based on a less rugged, enveloped HIV virus, and four plasmids are involved: a plasmid containing the gene(s) of interest and three packaging plasmids. In this case, however, there are no serotypes, so the same three packaging plasmids are used for every project. Aldevron has obtained rights to manufacture the set of plasmids developed by Oxford Genetics through a recent collaboration. These plasmids (pALD-Rev, pALD-VSV-G, pALD-GagPol and pALDLentiEGFP) have been optimized to produce high-titer, high-infectivity lentivirus. Optimization includes minimization of vector backbones, reduced homology with HIV/VSV and inter-cassette homology. In addition, lentiviral vectors produced with pALD Lenti packaging plasmids transfect cells at a higher rate than commercially available kits, with infectious titers in the range of 5 x 107 /mL. Once a client provides Aldevron with the gene of interest, it is cloned into the lentivirus expression plasmid (pALD-LentiEGFP) for custom production, replacing the EGFP sequence. The three packaging plasmids are available off the shelf. Aldevron’s production scale and experience with custom manufacturing of lentiviral expression plasmids, combined with the in-stock packaging plasmids, provide clients with a complete set of products and services for their programs. As with the AAV helper plasmid, the pALD lentivirus products are available royalty-free, and limited quantities of research material are provided free of charge. The products are currently available for research through commercial applications.
MANY BENEFITS FOR VECTOR MANUFACTURERS
AAV and LV manufacturing have traditionally required unique custom batches of helper and packaging plasmids, respectively. It can take 1-2 months for the production of these custom clinical and commercial plasmids. Aldevron’s off-the-shelf AAV and LV plasmids are, on the other hand, immediately and universally available with acceptable plasmid elements (i.e., kanamycin resistance). As a result, the timeline for production of AAV and LV vectors can be significantly reduced, accelerating project timelines and lowering cost. Cost is also reduced because these products do not carry any pass-through fees after purchase. As importantly, because Aldevron provides a reliable supply of consistent, high-quality plasmids and has demonstrated performance producing lentiviral and AAV vectors used in clinical trials, we facilitate supply chain risk reduction for our biopharma partners that are developing novel, life-changing therapies. Customers also benefit from access to three quality grades of standardized plasmids for use in research applications, the production of clinical trial materials and commercial GMP manufacturing. Clients have the option of choosing the quality grade that fits their needs and stage of development with the assurance that the royalty-free product will be available when it is needed.
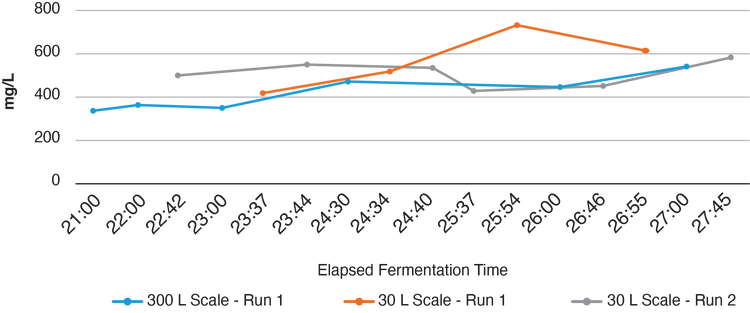
STRAIGHTFORWARD MANUFACTURING PROCESS
The bulk AAV and LV vectors from Aldevron are produced in Escherichia coli bacteria. First, the bacteria are subjected to a process that makes them competent (able to survive transient poration to allow the plasmids to enter), so they will be more accepting of the plasmid DNA. The bacteria are then transformed with the plasmid DNA. Exposure to antibiotic kills the bacteria that do not contain the plasmid. The remaining bacteria are grown and then lysed to release the plasmids. This step is challenging at large scale with large volumes, and Aldevron’s process development team has invested significant time and effort to develop large-scale processes that are highly efficient. The lysate is subjected to multiple filtration, chromatographic and buffer-exchange modalities before final formulation in the appropriate buffer to the desired concentration. This process can be performed at very small to large scales. Currently, Aldevron can grow bacteria to a volume of 300 liters in a single-use fermentor, which can contain as much as 100 g of DNA. Product characterization includes measurement of the supercoiling density and sequencing, among other analyses. The level of impurities (e.g., endotoxins, residual host-cell proteins, DNA and RNA) is also monitored. The basic attributes are dictated by the fact that these plasmids are used as raw materials for the production of viral vectors and are not intended for therapeutic applications that involve direct injection into humans.
MULTIPLE GRADES FIT FOR PURPOSE
The off-the-shelf plasmids from Aldevron are offered in three grades: research, GMP-Source® and GMP. Research-grade material is manufactured in a large, separate, open laboratory, in which multiple projects are performed simultaneously. The goal is to produce material rapidly at low cost and with high quality to enable effective research programs. Although the quality is high, it is not sufficient for use in humans because there is a small probability of contamination, even if at levels lower than can be detected. In addition, non-qualified assays are performed in a QC lab for these research materials. GMP material is produced in a new facility designed specifically for plasmid manufacturing — the largest plasmid manufacturing facility in the world. Multiple independent air-handling systems enable production of multiple lots simultaneously, with no two lots sharing the same HVAC. Extensive environmental monitoring and rigorous cleaning and changeover procedures help ensure the quality of the products. This material is produced in compliance with current Good Manufacturing Practices and is of sufficient quality to be injected directly into humans. GMP-Source material is manufactured in the same facility as our GMP products in separate suites that also have their own air-handling systems. The same raw materials are used for both GMP and GMP-Source materials, and all of the same qualified assays are used for both types of products and performed in a dedicated lab. The same quality system, which supports corrective actions and trending, is also used for both GMP and GMP-Source products. GMP-Source suites are not ISO classified, there is no environmental monitoring and the GMP-Source quality assurance review is limited to critical procedures. This quality level provides plasmids appropriate for use in viral vector production at a reduced cost and with a faster timeline without sacrificing any safety or efficacy in the final viral vector product. By offering this interim quality grade, we are helping viral vector manufacturers implement phase-appropriate GMP-compliant solutions.
| GMP-Source® | cGMP | |
| Screening for optimal growth conditions | ✔ | ✔ |
| Growth via shake flask or high-density fermentation | ✔ | ✔ |
| Alkaline lysis | ✔ | ✔ |
| Chromatographic purification | ✔ | ✔ |
| Consistent manufacturing process | ✔ | ✔ |
| Certificate of Analysis (CoA) | ✔ | ✔ |
| E. coli master cell bank generation | ✔ | ✔ |
| Manufacturing summary report | ✔ | ✔ |
| TSE/BSE statement | ✔ | ✔ |
| Master Batch Records dictate process | ✔* | ✔ |
| ISO classified fill/finish with environmental monitoring | ✔ | ✔ |
| QA oversight | ✔ | ✔ |
| Development and/or engineering work | ✔** | |
| Prescriptive change control process | ✔ | |
| Project specific master batch records | ✔** | |
| ISO classified production suites | ✔ |
*Batch record process controlled by Aldevron
**Optional services
ADVANCING THE FIELD
At Aldevron, we are driven to make a meaningful difference. We want to make real contributions to the lives of others by providing the basis for breakthroughs that improve human health and promote positive change throughout the world. As a CDMO, Aldevron offers a series of integrated science platforms for the provision of nucleic acid, protein and antibody services. By supplying our customers what they need, when they need it, with expert support at every stage of development and production, we help them open up their laboratories for ground breaking science and breakthrough discoveries. We are constantly looking to innovate and advance our technologies, products and services through partnerships with those who share our goal of advancing science in our field. We attach great importance to the close, collaborative relationships that allow us and our partners to concentrate on our core efforts. This means giving organizations of all sizes access to affordable, high-quality products and services to advance their work. We are also committed to bringing groups together to accelerate the process of drug and treatment discovery. In some cases, that requires changing traditional mindsets. With respect to our off-the-shelf plasmids for AAV and LV production, that means recognizing that some aspects of gene therapy and viral vaccine manufacturing do not need to be customized. Helper AAV and packaging LV plasmids do not require unique specifications that add complexity and cost. The off-the-shelf products Aldevron produces have specifications that are appropriate for viral vector manufacturing and are consistent with those applied to the production of viral vectors that have been administered to patients. Gene therapy development is highly complex, but identifying components that can be safely standardized allows innovation efforts to target other aspects that cannot be standardized. As the gene therapy field continues to grow and mature, we fully expect more players in the industry to realize — and leverage — the benefits offered by our off-the-shelf plasmids.
REFERENCES 1. Xiao, Xiao, Juan Li and Richard Jude Samulski. “Production of High-Titer Recombinant Adeno-Associated Virus Vectors in the Absence of Helper Adenovirus.” Journal of Virology. 72: 2224–2232 (1998).
