
Rep/Cap Plasmids
In Stock, Royalty-Free, Packaging Plasmid Solution for Recombinant AAV Manufacturing
pALD-AAV Rep/Cap Plasmids
Recombinant AAV (rAAV) manufacturing involves four key components, with the rep/cap plasmid being essential for viral genome replication and capsid formation. Choosing the right AAV serotype for your project is crucial for ensuring efficient and targeted gene delivery. Our pALD-AAV rep/cap plasmids are available with AAV serotype 2 replication protein expression and offer various capsid serotypes to provide optimal tissue tropism for your specific needs.
Our pALD-AAV rep/cap plasmids have several advantages:
- Royalty free, including for commercial products
- Kanamycin resistance
- Consistent, reliable supply
- Immediately available at research grade
With Aldevron's rep/cap plasmids, clients do not need to manufacture a custom batch as Aldevron maintains each as an in-stock product, reducing timeline and cost.

Each rep/cap plasmid contains the gene sequence for AAV serotype 2 replication (rep) protein paired with either serotype 2, 5 or 6 capsid (cap) protein respectively. Use of AAV serotype 2 rep proteins in combination with other serotypes for cap proteins is referred to as pseudotyping which provides enhanced and refined tissue tropism in some applications.
| Plasmid | Rep Serotype | Cap Serotype | Pack Size |
| pALD-AAV2 | 2 | 2 | 10 mg |
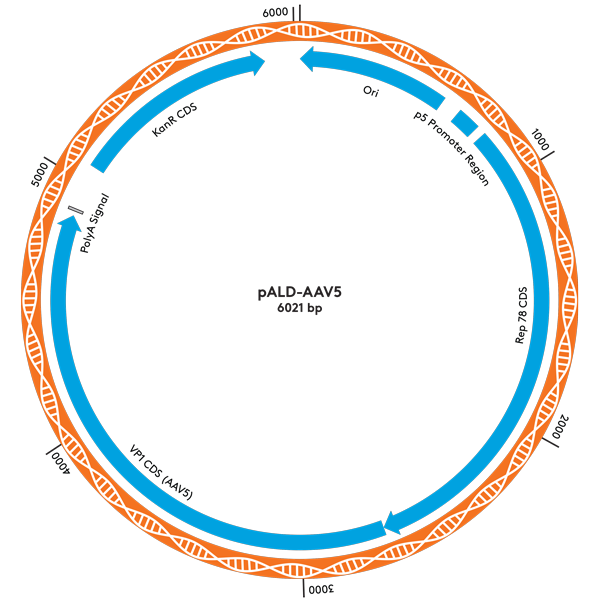
| pALD-AAV5 | 2 | 5 | 10 mg |
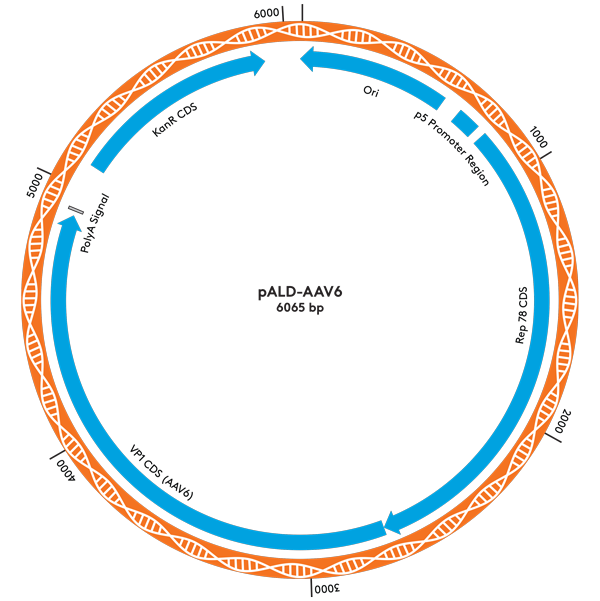
| pALD-AAV6 | 2 | 6 | 10 mg |
Bulk quantities and samples are available upon request.
Plasmid Maps

| Appearance | Clear and Colorless |
| Buffer | TE |
| USP Sterility <71> * | No Growth |
| Concentration | 1.0 mg/mL +/- 10% |
| DNA Homogeneity | ≥ 80% Supercoiled |
| Endotoxin | ≤ 100 EU/mg |
| Identity | Confirmed Against DNA Ladder |
| Plasmid Identity | Identical to Reference Sequence |
| ABS 260/280 Ratio Purity | 1.80 - 2.00 |
| Residual Host Genomic DNA via qPCR | ≤ 5% |
| Residual Host Protein via BCA | ≤ 2% |
| Residual Host RNA via SYBR Gold | ≤ 5% by Semi-Quantitative Densitometry |
| Restriction Digest | Matches Expected Restriction Pattern |
| Mycoplasma Contamination | Negative for the Presence of Mycoplasma |
*Sterility testing is not available for research grade material. Bioburden by direct inoculation with a specification of no growth after 48 hours is performed at the research grade level.