
pALD-ITR Plasmids
Unlock Your rAAV Potential with our In Stock, Royalty-Free, Expression Plasmids
We offer ready-to-use, royalty-free expression plasmids specifically designed for recombinant AAV (rAAV) manufacturing. Our pALD-ITR GFP reporter plasmids and pALD-ITR cloning vectors feature the CMV promoter and enhancer, ensuring reliable and efficient expression of therapeutic genes for your gene therapy research and development needs.
pALD-ITR GFP Reporters:
Aldevron's pALD-ITR GFP reporter plasmids have several advantages:
- Royalty free for research use
- Kanamycin resistance
- Available with and without WPRE
- Consistent, reliable supply
Immediately available at research grade


pALD-ITR Cloning Vectors:
Aldevron's pALD-ITR cloning vectors have several advantages:
- Royalty free for research through commercial use
- Kanamycin resistance
- Available with and without WPRE
- Available off the shelf for cloning and gene synthesis
- Immediately available at research grade
Aldevron's pALD-ITR GFP reporter plasmids and cloning vectors are available as free samples. If you are interested, please fill out the request sample form.
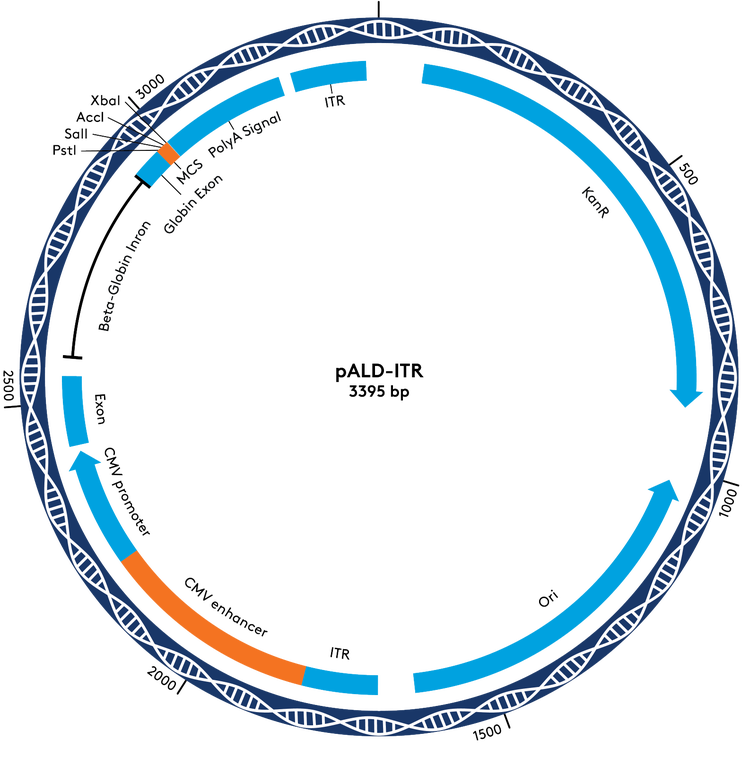
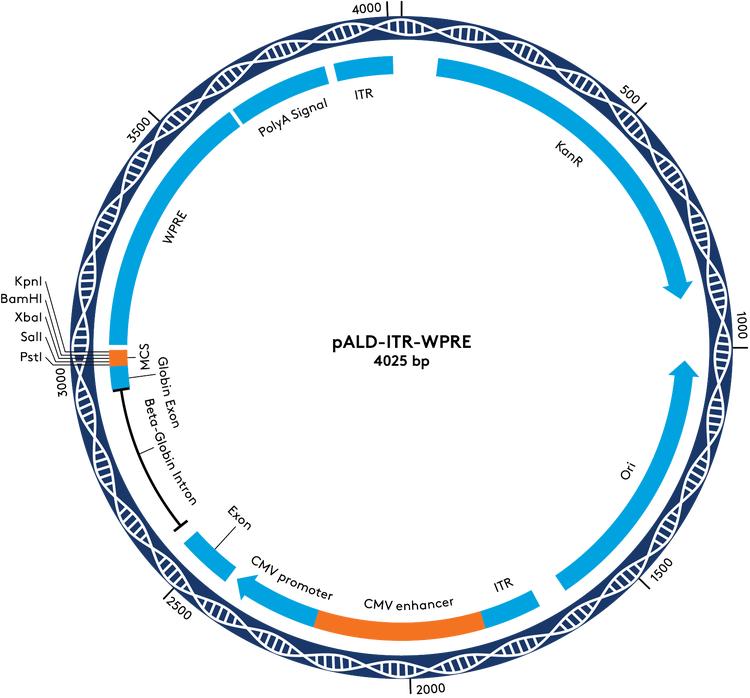
Ordering Information
pALD-ITR GFP Reporters Quality Control Specifications
| Appearance | Clear and Colorless |
| Buffer | TE |
| Bioburden | No growth in 48 hours at 37 +/- 2 C |
| Concentration | 1.0mg/mL +/- 10% |
| DNA Homogeneity | ≥80% Supercoiled |
| Endotoxin | ≤100 EU/mg |
| Identity | Confirmed Against DNA Ladder |
| Plasmid Identity | Identical to Reference Sequence |
| ABS 260/280 Ratio Purity | 1.80 – 2.00 |
| Residual Host Genomic DNA via qPCR | ≤5% |
| Residual Host Protein via BCA | ≤2% |
| Residual Host RNA via SYBR Gold | ≤5% by Semi-Quantitative Densitometry |
| Restriction Digest | Matches Expected Restriction Pattern |
| Mycoplasma Contamination | Negative for the Presence of Mycoplasma |
pALD-ITR Cloning Vectors Quality Control Specifications
| Appearance | Clear and Colorless |
| Buffer | TE |
| Concentration | 1.0mg/mL +/- 10% |
| DNA Homogeneity | ≥80% Supercoiled |
| Endotoxin | ≤100 EU/mg |
| Identity | Confirmed Against DNA Ladder |
| Plasmid Identity | Identical to Reference Sequence |
| ABS 260/280 Ratio Purity | 1.80 – 2.00 |
| Residual Host Genomic DNA | ≤5% |
| Residual Host RNA | Not Visible at 200 ng Load |
| Restriction Digest | Matches Expected Restriction Pattern |