
Plasmid DNA Production & Manufacturing Services
Plasmid DNA for research, preclinical, and clinical applications scaled to fit projects of any size
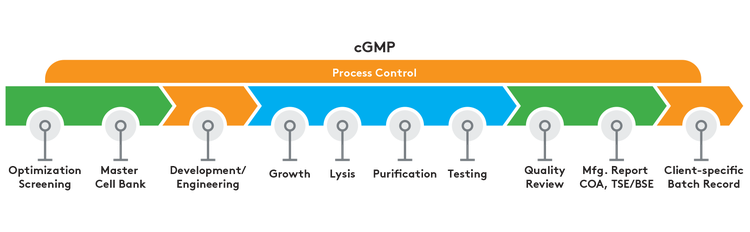
Aldevron has been perfecting plasmid DNA production for more than 20 years, using proprietary technology to manufacture DNA for a wide range of research, preclinical, clinical, and diagnostic applications.
Whether you require 1mg of DNA for a research study or 100 g for a clinical trial, our goal is to provide you with the most affordable, high-quality plasmid DNA manufacturing services that meet your requirements.
Research-Grade Plasmid DNA
We manufacture high-quality plasmid DNA, linearized vectors, and thousands of preparations per day for high-throughput screening.
GMP-Source® Plasmid DNA
Discover how GMP-Source® plasmid DNA retains the key components of cGMP manufacturing to offer a faster and cost-effective alternative to GMP plasmids.
GMP Plasmid DNA
Our customized GMP plasmid DNA manufacturing includes a comprehensive quality assurance process, ensuring each order contains the highest quality products.
Nanoplasmid™ Platform
Ideally suited for gene therapy applications. Nanoplasmid DNA is customized for each client.
Catalog Plasmid DNA
Catalog items to assist you in your research. Many are in-stock and ready to ship immediately.
Applications
Transfection
Pure, high-quality plasmid DNA is critical for a successful transfection. Our high-purity, high-homogeneity, low-endotoxin plasmid DNA is ideal for any transfection application.
Case Study: A client suffered from very low plasmid production yields using other vendors. Our optimization resulted in a 9X increase in the yield per liter of media. In addition, the purity far exceeded that of previous manufacturing attempts.
Gene therapy
From ITR-bearing cis-plasmids to very large helper plasmids, our manufacturing technology produces high yields even for the most difficult constructs.
Case Study: A client required 95% monomer plasmid DNA as the final deliverable. The starting material provided was predominantly dimerized/multimeric. Our team linearized the material, ligated it back together to create the monomer, and transformed. We achieved the required specification for both the supercoiled and linearized plasmid DNA deliverable.
In vivo studies for DNA vaccines
With a consistent manufacturing process from research grade through clinical production, we ensure reliable, high-quality DNA for your vaccine program.
Case Study: When West Nile virus threatened the survival of endangered condors in California, we were contracted to manufacture a DNA vaccine developed by the Centers for Disease Control and Prevention. Our production and quality control systems allowed us to manufacture the vaccine in less than two months. The vaccine went on to protect other endangered species against West Nile virus.
RNA template
Whether your application is a messenger RNA therapeutic or guide RNA for CRISPR-Cas9, we have extensive experience producing linearized plasmid DNA using a variety of enzymes with advanced purification techniques.
Case Study: A client required a high yield and consistent poly(A) tail length for their template. We evaluated a variety of resin and buffer combinations as well as post-linearization purification strategies. The result was 90-95% recovery of linearized DNA with a consistently high poly(A) tail length.