
Custom Plasmid DNA Manufacturing
Aldevron delivers confidence
When working with plasmids, nothing is certain. Every plasmid is unique, from Nanoplasmid™ vectors to traditional plasmid constructs. Variations in promoters, antibiotic resistance cassettes, secondary structures (such as ITRs) and differing copy numbers can lead to challenges scaling up to clinical or commercial production, etc.
Aldevron has been balancing the science and art of plasmid manufacturing for 25 years, generating more than 165,000 lots, 5,000 at clinical grade, and supporting multiple commercial programs. When working on complex cell, gene, and mRNA-based therapies and vaccines, rely on our experience with plasmid production, so you can focus on developing therapies that can transform patients’ lives.
Supporting progress in multiple dimensions
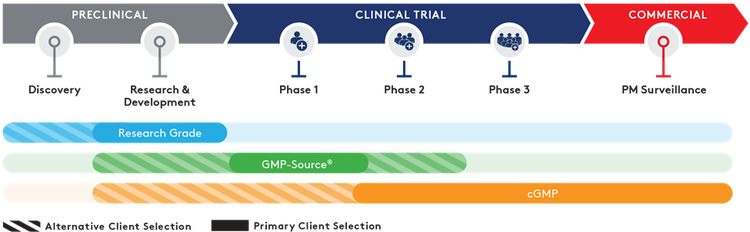
Quality grades
Working with multiple partners can increase costs and risk delays with difficult and time-consuming technical transfers. Aldevron offers phase-appropriate quality grades – from research to Small-Scale and traditional cGMP – that are purposefully designed as a pathway from research to potential clinical success and commercialization.
- Research Grade – Fast turn-around, customizable dispensing, mg-to-g quantities
- GMP-Source™ Grade – Cost-effective adoption of relevant cGMP manufacturing features
- Small-Scale and Traditional cGMP Grade – Highest quality oversight, process control, segregated ISO-classified manufacturing suites with phase and scale-appropriate cGMP services.
| The Aldevron Process | Research Grade | GMP- Source | cGMP |
| Screening for optimal growth conditions | ✔ | ✔ | ✔ |
| Growth via shake flask or highfermentation | ✔ | ✔ | ✔ |
| Alkaline Iysis | ✔ | ✔ | ✔ |
| Chromatographic purification | ✔ | ✔ | ✔ |
| Consistent manufacturing process | ✔ | ✔ | ✔ |
| Certificate of Analysis (CoA) | ✔ | ✔ | ✔ |
| E.coli master cell bank generation | ✔ | ✔ | |
| Manufacturing summary report | ✔ | ✔ | |
| TSE/BSE statement | ✔ | ✔ | |
| Validated QA oversight | ✔ | ✔ | |
| Development and/or engineering work | ✔* | ||
| Validated changes control systems | ✔ | ||
| Project specific master batch records | ✔* | ||
| ISO classified production suites | ✔ | ||
| Environmental monitoring | ✔ |
*Optional services
Production scale
Our plasmid DNA manufacturing happens across 3 different facilities, ranging from high-throughput, small scale preps (<1mg) for research and pre-clinical activities to hundreds of grams for commercial applications. Aldevron uses a variety of optimization tools to increase efficiency and reduce costs for the manufacturing needs of our partners. We utilize shake flasks and a variety of bioreactors, including single-use fermenters, to meet our clients’ needs throughout their journey. Roughly this translates to:
- Research Grade: 1 mg – 20g
- GMP-Source™
- Small-Scale cGMP Plasmid ≤500mg
- Traditional cGMP Plasmid >500mg – hundreds of grams
Optimization tools
When working on basic research, plasmids are easy – transform, grow, lyse, bind, wash, and elute. When a plasmid is a critical raw material for a therapeutic or vaccine there’s quite a bit more to it. Cost control becomes a key factor. Increasing yield and/or reducing manufacturing runs can cut costs. Plasmid performance, such as increasing viral titers in viral vector applications, could further drive down costs. After 25 years, we have a full toolbox to optimize plasmid production, including but not limited to:
- Nanoplasmid vectors – Modern alternative to traditional plasmids featuring increased manufacturing yields, high transgene expression and an antibiotic-free selection system.
- REVIVER™ Host Strains – Engineered specifically for stable, high-yield production of poly(A), ITR, and large palindrome-containing traditional plasmids.
- HYPER-GRO™ Manufacturing Platform – Advanced, fed-batch, nutrient-limited process that can reach yields of up to 3.5 g/L.
- Aldevron Storage Distribution Services (ASDS) - A secure, monitored facility for storage and shipment of research-grade, GMP-Source, and cGMP biological materials produced by Aldevron.
- Pre-banking – Allows for evaluation of the sequence integrity prior to manufacturing of the master cell bank. Learn more in the article, “Pre-banking and Avoiding Manufacturing Challenges for GMP Plasmids Containing Unstable Sequence Regions.”
Standardized Products
Aldevron has created a suite of standardized, stocked products that can create efficiencies and move your project to clinical trials and commercialization more efficiently. From increased knowledge of production to streamlining/eliminating licensing concerns, standard products can simplify projects. Learn more in the Breakthrough Blog – The Benefits of Streamlining and Standardizing Plasmid DNA for Cell and Gene Therapies.
- WEBINAR: Advanced plasmid technology: improving safety and performance
- WEBINAR: Can a Small Plasmid Produce Huge Benefits? The Power of Small
- WHITEPAPER: Next Generation Plasmid Technology: Improving Performance, Safety, and Manufacturing for Today’s Therapies
- WHITEPAPER: Eliminating antibiotic resistance gene transfer risks in cell & gene therapies: Nanoplasmid Vectors.
- WHITEPAPER: Streamlining and Standardizing Cell and Gene Therapies from Process to Product
- WHITEPAPER: Gene and Cell Therapy: Planning for Manufacturing Success Early
- BROCHURE: Plasmid DNA Manufacturing
- BROCHURE: Nanoplasmid™ Vector Platform
When you start your research with us, you can travel one continuous path to finished product. We’ve designed and built facilities and capabilities to meet today’s needs, while continuing to build for tomorrow. Our most recent addition is LNP formulations, with our partners at Precision Nanosystems, and sterile fill finish capabilities to deliver drug product.

|

|

|
| Breakthrough Campus | Advance Campus | Aldevron Madison |
| Fargo, ND | Fargo, ND | Madison, WI |
|
Plasmid DNA, RNA, Protein manufacturing at cGMP & GMP-Source™ quality for clinical applications GMP Facility: 259,000 SF |
Plasmid DNA manufacturing for research applications; Process development Facility: 45,000 SF |
Protein manufacturing and process development for research and diagnostics Facility: 52,000 SF |
| Learn More | Learn More | Learn More |
Talk with our experts
Interested in working together or have questions about our products and services? Reach out to our client relations team to learn more about what we offer and how to get started on your next project.
| region | na1 |
| portalId | 1769030 |
| formId | c799884c-a1bd-4948-8cfe-2cf904b67d66 |
| target | Services-hubspot-form |


